Single Replacement Worksheet Answers
Single Replacement Worksheet Answers - For transition metals use the following charges: Zn + hcl zncl 2 + h 2 type of reaction: Web fe + cu(oh)2 → write the reactions and predict the products of each of the following single replacement reactions. If no reaction occurs write n.r. Cl 2 + ki → 4. The starting materials are always pure elements like hydrogen gas or a pure zinc metal, plus an aqueous compound.
A + bc → b + ac or a + bc → c + ba (when a and c are negative ions) 1. + bc → ac + b. Students will learn to use the activity series for the first time as part of this worksheet, as. Use single replacement reactions to create voltage (make a battery). A + bc → ac + b a + bc → ac + b.
Web single replacement reaction worksheets 2024. Web single and double replacement reactions practice ws coleman; The starting materials are always pure elements like hydrogen gas or a pure zinc metal, plus an aqueous compound. Write correct formulas for the products in these single replacement reactions. Web single replacement reaction worksheet key | pdf | chemical compounds | sets of chemical elements.
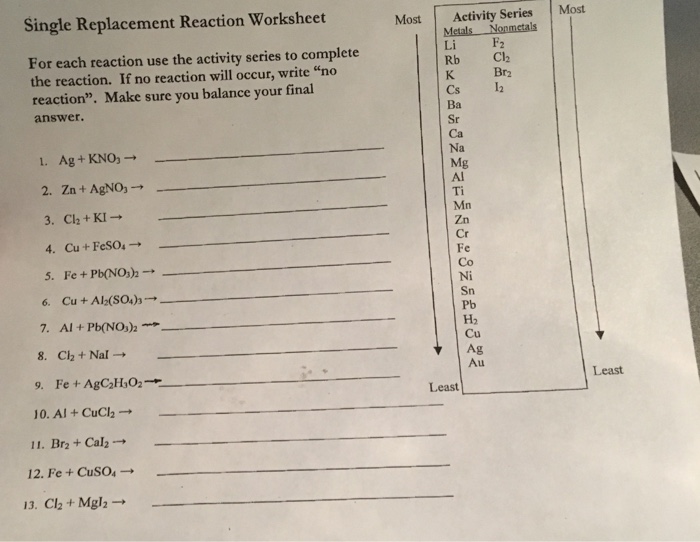
Ag + kno 3 → 2. Web worksheet on single & double replacement reactions predict the products. Cu + feso 4 → 5. Web chemistry single replacement reaction worksheet. Using the activity series table, complete the following reactions by writing the products that are formed.
Web chemistry single replacement reaction worksheet 1. Using the activity series table, complete the following reactions by writing the products that are formed. A + bc → b + ac or a + bc → c + ba (when a and c are negative ions) 1. Fe + pb(no 3) 2 → 6. Web fe + cu(oh)2 → write the.
Make sure you balance your final answer. The starting materials are always pure elements like hydrogen gas or a pure zinc metal, plus an aqueous compound. Li is above mg on the chart 2. Get examples of single replacement reactions and learn how to use the metal reactivity series to predict whether a reaction will occur and the products. A.
Using the activity series table, complete the following reactions by writing the products that are formed. If no reaction will occur, write “no reaction”. In single replacement reactions, one element reacts with a compound by replacing one of the elements within the compound. In this general reaction, element a a is a metal and replaces element b b (also a.
Web single replacement reaction worksheet for each reaction use the activity series to complete the reaction. Web single replacement reaction worksheets 2024. If no single replacement reaction occurs, write nr to the right of the arrow. React metals with salts of other metals to cause a single replacement reaction. Web fe + cu(oh)2 → write the reactions and predict the.
If the reaction will not occur, write “no reaction.” • if the reaction occurs, write the correct formulas for the reactants and products. + bc → ac + b. In single replacement reactions, one element reacts with a compound by replacing one of the elements within the compound. In this general reaction, element a a is a metal and replaces.
Fe + pb(no 3) 2 → 6. Ag + kno 3 → 2. Metal can replace a metal ion in a salt, or a hydrogen ion in an acid: List metals in order of activity based on your observation. For transition metals use the following charges:
Single Replacement Worksheet Answers - Li is above mg on the chart 2. Web worksheet on single & double replacement reactions predict the products. The general equation is a + bc → ac + b examples are zn + 2hcl → zncl₂ + h₂, where zn replaces h in hcl, and f₂. List metals in order of activity based on your observation. If there is no reaction, then just put no rxn. Cl 2 + ki → 4. + bc → ac + b. Write formulas & balance each reaction. Get examples of single replacement reactions and learn how to use the metal reactivity series to predict whether a reaction will occur and the products. If no reaction will occur, write “no reaction”.
Write formulas & balance each reaction. Web get the definition of a single replacement reaction or single displacement reaction. React metals with dilute acid to produce hydrogen gas. List metals in order of activity based on your observation. Web worksheet on single & double replacement reactions predict the products.
Predicting single replacement reactions for each of the following reactions, • predict whether or not the reaction will occur. Web single replacement reaction worksheet for each reaction use the activity series to complete the reaction. React metals with dilute acid to produce hydrogen gas. These high school chemistry worksheets are full of pictures, diagrams, and deeper questions covering all aspects of chemical reactions!
If there is no reaction, then just put no rxn. Cl 2 + ki → 4. The general equation is a + bc → ac + b examples are zn + 2hcl → zncl₂ + h₂, where zn replaces h in hcl, and f₂.
Carry out single replacement reactions. Use single replacement reactions to create voltage (make a battery). + bc → ac + b.
Students Will Learn To Use The Activity Series For The First Time As Part Of This Worksheet, As.
These high school chemistry worksheets are full of pictures, diagrams, and deeper questions covering all aspects of chemical reactions! Web chemistry single replacement reaction worksheet. Cl 2 + ki → 4. Web single and double replacement reactions practice ws coleman;
If No Reaction Occurs Write N.r.
In single replacement reactions, one element reacts with a compound by replacing one of the elements within the compound. Fe + pb(no 3) 2 → 6. Web single replacement reaction worksheet key | pdf | chemical compounds | sets of chemical elements. Magnesium (mg) replaces hydrogen (h) in hydrochloric acid (hcl) and forms magnesium chloride (mgcl 2) and gaseous hydrogen (h 2 ).
Using The Activity Series Table, Complete The Following Reactions By Writing The Products That Are Formed.
Get examples of single replacement reactions and learn how to use the metal reactivity series to predict whether a reaction will occur and the products. Metal can replace a metal ion in a salt, or a hydrogen ion in an acid: Ca 3 n 2 + h 2 o ca(oh) 2 + h 3 n type of reaction: Use the activity series to determine this.
This Unit Is Meant To Cover The Basics Of Chemical Reactions (Combustion, Synthesis, Decomposition, Single Replacement, Double Replacement) Along With Solubility And Metal Reactivity.
+ bc → ac + b. Make sure you balance your final answer. Carry out single replacement reactions. The general equation is a + bc → ac + b examples are zn + 2hcl → zncl₂ + h₂, where zn replaces h in hcl, and f₂.