Isotopes Worksheet Answers
Isotopes Worksheet Answers - 6 protons & 6 neutrons e. 19 protons, 22 neutrons, 19 electrons. What do all isotopes of an element have in common? 5 protons, 6 neutrons, 5 electrons. (i) 4019 p (ii) 12050 sn (iii) 2963 cu (iv) 10947 au (v) 5826 fe. For example, the atomic mass of carbon is reported as 12.011 amu (atomic mass units).
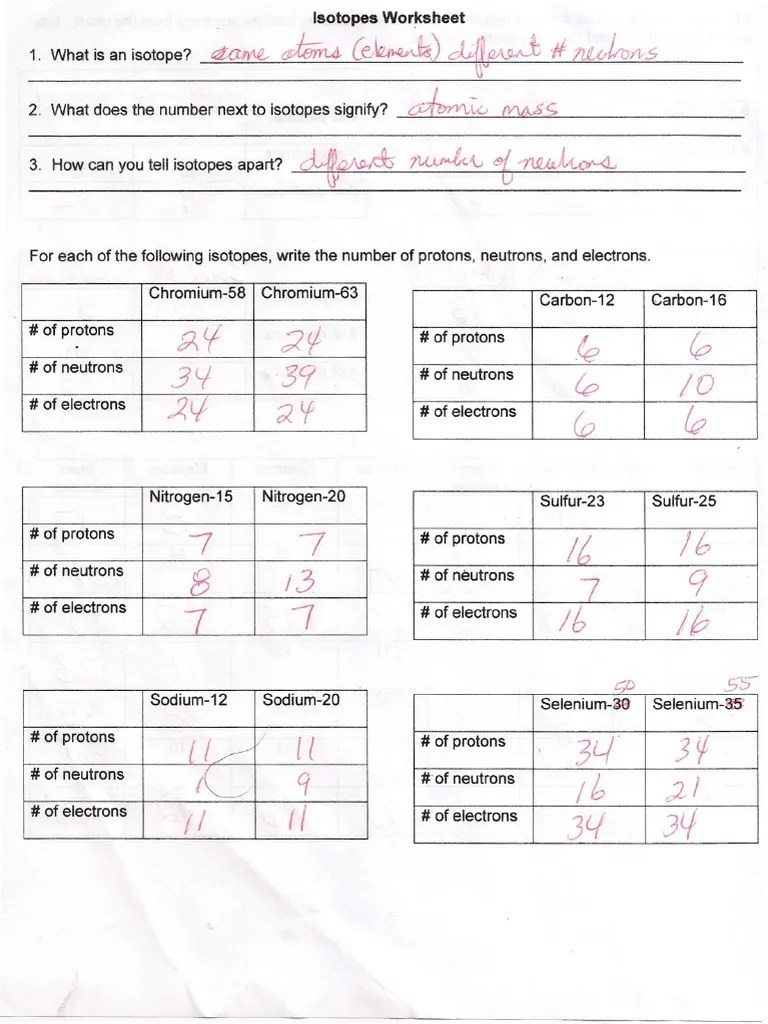
What does the number next to isotopes signify? The number 6 refers to the _________________________ c. _____ _____ for each of the following isotopes, write the # of protons, neutrons, and electrons. Here are three isotopes of an element: Identify which among the isotope symbols below is incorrect.
The numbers 12, 13, and 14 refer to the mass number. 12c 14 13c c a. Full written answers and a video explanation for this worksheet is also available. \textcolor {f21cc2} {\text {number of neutrons}=\text {mass number. Which isotope of lead is likely to be the most abundant?
Give a reason for your answer. Web isotopes aqa gcse. 12c 14 13c c a. Web isotopes worksheets and full answers. The number 6 refers to the atomic number.
Use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element. The numbers 12, 13, and 14 refer to the mass number. How can you tell isotopes apart? Here are three isotopes of an element: How can you tell one isotope from another?
How can you tell isotopes apart? Imagine you have 90 balls with mass 200 g, and 10 balls with mass 300 g. The average mass of the balls is given by: The numbers 12, 13, and 14 refer to the mass number. How can you tell one isotope from another?
How many protons and neutrons are in the first. How many protons and neutrons are in the first isotope? \textcolor {f21cc2} {\text {number of neutrons}=\text {mass number. Here are three isotopes of an element: _____ _____ for each of the following isotopes, write the # of protons, neutrons, and electrons.
Exshare answers from the class, ensuring that all students are able to press 18o/16o in terms of voltage and resistance before allowing them to move on to calculate 18o/16o ratio from the data provided. The numbers 12, 13, and 14 refer to the mass number. The number 6 refers to the atomic number. It includes a series of questions of.
Worksheets for a lesson on isotopes, including a full set of answers on powerpoint slides. Which isotope of lead is likely to be the most abundant? Makes an effective homework or revision resource including a challenge question to stretch higher ability students. Web pdf, 1.36 mb. For each of the following isotopes, write the number of protons, neutrons, and electrons.
The average mass of the balls is given by: _____ _____ for each of the following isotopes, write the # of protons, neutrons, and electrons. Identify which among the isotope symbols below is incorrect. Web isotopes aqa gcse. Web ask the class to read through the remaining sections on the provided worksheet, and answer the questions.
Isotopes Worksheet Answers - If we know the number mass number and the atomic number, we can calculate the number of neutrons in the atom using: \textcolor {f21cc2} {\text {number of neutrons}=\text {mass number. The number 6 refers to the _________________________ c. Calculate the average atomic mass of chlorine if its isotopes and % abundances are as follows. Identify which among the isotope symbols below is incorrect. How can you tell isotopes apart? A short worksheet to introduce or revise isotopes. One isotope has a mass number of 10 and the other isotope has a mass number of 11. (i) 4019 p (ii) 12050 sn (iii) 2963 cu (iv) 10947 au (v) 5826 fe. How many protons and neutrons are in the first.
The number 6 refers to the atomic number. 5 protons, 6 neutrons, 5 electrons. Web fill in the blanks with the correct answers for the following: How can you tell isotopes apart? What do all isotopes of an element have in common?
Give a reason for your answer. Calculate the average atomic mass of chlorine if its isotopes and % abundances are as follows. The numbers 12, 13, and 14 refer to the mass number. Students shared 1007 documents in this course.
Here are three isotopes of an element: The number 6 refers to the atomic number c. 19 protons, 22 neutrons, 19 electrons.
The average atomic mass of a lead atom is 207.2 amu. If we know the number mass number and the atomic number, we can calculate the number of neutrons in the atom using: Web atoms of the same element with different numbers of neutrons are called isotopes.
Web In A Sample Of E There Are Two Isotopes.
Isobars are nuclides of different elements (different \(z\)) with the same mass number (\(a\)). Web fill in the blanks with the correct answers for the following: Consider the following illustrations in which protons are shown as pink spheres and neutrons are shown as green spheres. So different isotopes have different mass numbers but the same proton number.
Use The Sim To Learn About Isotopes And How Abundance Relates To The Average Atomic Mass Of An Element.
Exshare answers from the class, ensuring that all students are able to press 18o/16o in terms of voltage and resistance before allowing them to move on to calculate 18o/16o ratio from the data provided. Great practice for students to master atomic and mass numbers. Web isotopes practice set oe aa — 1. Worksheets for a lesson on isotopes, including a full set of answers on powerpoint slides.
Here Are Three Isotopes Of An Element:
What does the number next to isotopes signify? The average mass of the balls is given by: Web ask the class to read through the remaining sections on the provided worksheet, and answer the questions. Here are three isotopes of an element:
This Worksheet Is Designed For Gcse Physics Students.
The number 6 refers to the _________________________ c. The numbers 12, 13, and 14 refer to the ________________________ d. The number 6 refers to the atomic number. If we know the number mass number and the atomic number, we can calculate the number of neutrons in the atom using: