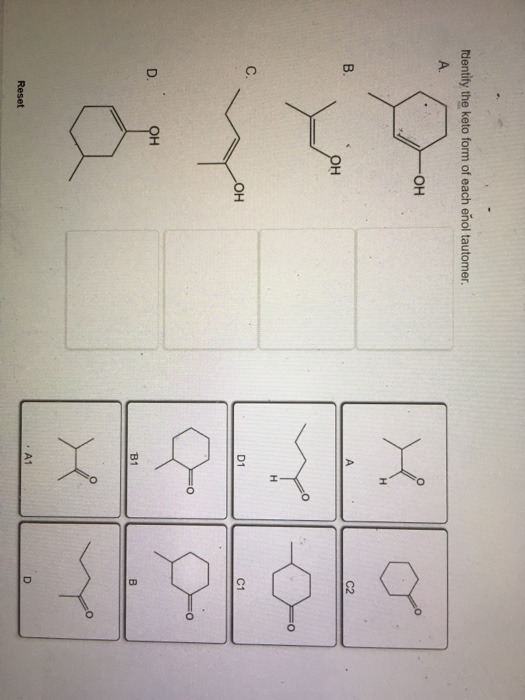
Identify The Keto Form Of Each Enol Tautomer
Identify The Keto Form Of Each Enol Tautomer - Often, the position of carbon atoms near a carbonyl group are designated by greek letters. You will use nmr spectroscopy to determine the equilibrium constant for the reaction, and then investigate the reasons for any differences in the equilibrium constants via computational methods. For alkylation reactions of enolate anions to be useful, these intermediates must be generated in high concentration in the absence of other strong nucleophiles and bases. Identify the keto form of each enol tautomer. Web the individual keto and enol isomers are called tautomers. The mechanistic return to the keto tautomer begins with deprotonation of the hydroxyl hydrogen to produce an enolate ion.
You will use nmr spectroscopy to determine the equilibrium constant for the reaction, and then investigate the reasons for any differences in the equilibrium constants via computational methods. The α hydrogen is then deprotonated to form the enol tautomer. One applies to acidic conditions, while the other applies to basic conditions. Identify the keto form of each enol tautomer. The ketone or aldehyde is generally strongly favored in this reaction.
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web in a solution, you won't see much of the enol form, but these can occur. The ketone or aldehyde is generally strongly favored in this reaction. For alkylation reactions of enolate anions to be useful, these intermediates must be generated in high concentration in the absence of other strong nucleophiles and bases. Make certain that you can define, and use in context, the key terms below.
Note the difference between tautomers and resonance forms. It can spontaneously through equilibrium get to the actual enol form. The oxonium ion undergoes resonance stabilization by moving the π electrons from the c=o bond towards the protonated oxygen. Web the individual keto and enol isomers are called tautomers. Identify the keto form of each enol tautomer.
Note the difference between tautomers and resonance forms. Web thus, tautomerism describes the equilibrium between keto and enol forms interconverted through a change in the position of bonding electrons and hydrogen to produce two isomers. Uncover how aldehydes or ketones, with a dash of acid or base, can transform into an enol. Then lone pair electrons from the enolate anion.
Identify the keto form of each enol tautomer. It can spontaneously through equilibrium get to the actual enol form. There are two distinct reaction pathways to be considered since the presence of either acidic or basic conditions leads to tautomerism. Both the reactions involve the same steps—protonation and deprotonation— although in. The ketone or aldehyde is generally strongly favored in.
View the full answer step 2. The ketone or aldehyde is generally strongly favored in this reaction. Both the reactions involve the same steps—protonation and deprotonation— although in. Often, the position of carbon atoms near a carbonyl group are designated by greek letters. Of the spectral line width.
Enol and enolate are two related chemical species that are commonly encountered in organic chemistry. Make certain that you can define, and use in context, the key terms below. For alkylation reactions of enolate anions to be useful, these intermediates must be generated in high concentration in the absence of other strong nucleophiles and bases. You will use nmr spectroscopy.
The chemical equilibrium is thermodynamically driven. Both the reactions involve the same steps—protonation and deprotonation— although in. So, what is an enol? Web the individual keto and enol isomers are called tautomers. Enol tautomer → keto tautomer.
Both the reactions involve the same steps—protonation and deprotonation— although in. Because carbonyl groups are sp 2 hybridized the carbon and oxygen both have un hybridized p orbitals which can overlap to form the c=o π π bond. Then lone pair electrons from the enolate anion attack an electrophilic h + through conjugation with the double bond. Ldentify the enol.
Identify The Keto Form Of Each Enol Tautomer - Of the spectral line width. The α hydrogen is then deprotonated to form the enol tautomer. Web the keto tautomer adopts a planar geometry in the d0 state similar to that in the neutral ground state. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Then lone pair electrons from the enolate anion attack an electrophilic h + through conjugation with the double bond. The mechanistic return to the keto tautomer begins with deprotonation of the hydroxyl hydrogen to produce an enolate ion. Not the question you’re looking for? One applies to acidic conditions, while the other applies to basic conditions. You will use nmr spectroscopy to determine the equilibrium constant for the reaction, and then investigate the reasons for any differences in the equilibrium constants via computational methods. Web thus, tautomerism describes the equilibrium between keto and enol forms interconverted through a change in the position of bonding electrons and hydrogen to produce two isomers.
Web thus, tautomerism describes the equilibrium between keto and enol forms interconverted through a change in the position of bonding electrons and hydrogen to produce two isomers. Then lone pair electrons from the enolate anion attack an electrophilic h + through conjugation with the double bond. Note the difference between tautomers and resonance forms. Web the individual keto and enol isomers are called tautomers. It can spontaneously through equilibrium get to the actual enol form.
Web the keto and enol forms are known as tautomers and they constantly interconvert (or tautomerize) between the two forms under acid or base catalyzed conditions. There are two distinct reaction pathways to be considered since the presence of either acidic or basic conditions leads to tautomerism. Of the spectral line width. View the full answer step 2.
Web the individual keto and enol isomers are called tautomers. Topics covered in other articles. Web the keto tautomer adopts a planar geometry in the d0 state similar to that in the neutral ground state.
Web the keto and enol forms are known as tautomers and they constantly interconvert (or tautomerize) between the two forms under acid or base catalyzed conditions. In addn., no spectroscopic evidence for fast proton transfer from the enol form to the keto form in the d0 state has been obtained from the anal. Web 2) protonation the enolate ion to form an enol.
It Can Spontaneously Through Equilibrium Get To The Actual Enol Form.
The α hydrogen is then deprotonated to form the enol tautomer. Note the difference between tautomers and resonance forms. The oxonium ion undergoes resonance stabilization by moving the π electrons from the c=o bond towards the protonated oxygen. Tautomers are constitutional isomers—different compounds with different structures—while resonance forms are different representations of a single compound.
Web Thus, Tautomerism Describes The Equilibrium Between Keto And Enol Forms Interconverted Through A Change In The Position Of Bonding Electrons And Hydrogen To Produce Two Isomers.
Web the individual keto and enol isomers are called tautomers. Both the reactions involve the same steps—protonation and deprotonation— although in. Topics covered in other articles. Enol and enolate are two related chemical species that are commonly encountered in organic chemistry.
There Are Two Distinct Reaction Pathways To Be Considered Since The Presence Of Either Acidic Or Basic Conditions Leads To Tautomerism.
For alkylation reactions of enolate anions to be useful, these intermediates must be generated in high concentration in the absence of other strong nucleophiles and bases. Web 2) protonation the enolate ion to form an enol. Often, the position of carbon atoms near a carbonyl group are designated by greek letters. In this article, you will learn about keto enol tautomerization, including the meaning of “tautomerization,” trends in enol stability, and important mechanisms under acidic and basic conditions.
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
Web the individual keto and enol isomers are called tautomers. Web the individual keto and enol isomers are called tautomers. You will use nmr spectroscopy to determine the equilibrium constant for the reaction, and then investigate the reasons for any differences in the equilibrium constants via computational methods. Note the difference between tautomers and resonance forms.