How Does Pyrite Form
How Does Pyrite Form - These compounds were formed after igneous rocks are pressurized, heated, and subsequently changed over millions of years. It has a chemical composition of iron sulfide (fes 2) and is the most common sulfide mineral. Web for additional information, see the gemstone section on pyrite. Pyrite forms under reducing conditions, typically in environments with low oxygen levels, high sulfur content, and abundant iron. Web pyrite typically forms in cubic crystals, often accompanied by striations on the crystal faces. Iron sulfide, sometimes containing small amounts of cobalt, nickel, silver, and gold.
Web pyrite, also known as “fool’s gold,” is a common iron sulfide mineral with the chemical formula fes2. The name comes from the greek word pyr, “fire,” because pyrite emits sparks when struck by metal. Howstuffworks | aug 25, 2023. One unexpected consequence of the model is that it further explains how commonly observed groups of framboids can form contemporaneously. This remarkable mineral often occurs in sedimentary rocks, coal beds, shale, and sometimes in hydrothermal veins or igneous rocks.
Slower cooling results in larger crystals. Pyrite is called fool’s gold; The name comes from the greek word pyr, “fire,” because pyrite emits sparks when struck by metal. These chlorite compounds contain the iron found in pyrite, but they also contain silicone and oxygen, which must be removed and. Commonly called fool’s gold, pyrite is the.
These chlorite compounds contain the iron found in pyrite, but they also contain silicone and oxygen, which must be removed and. Web pyrite, also known as “fool’s gold,” is a common iron sulfide mineral with the chemical formula fes2. It forms in a variety of geological settings through several processes. Iron sulfide, sometimes containing small amounts of cobalt, nickel, silver,.
Slower cooling results in larger crystals. It has a chemical composition of iron sulfide (fes 2) and is the most common sulfide mineral. Web pyrite, a naturally occurring iron disulfide mineral. Web how does pyrite form (pyritisation)? Bisulphite can be partially oxidised or can react with organic matter and reactive metal species.
Web pyrite typically forms in cubic crystals, often accompanied by striations on the crystal faces. These chlorite compounds contain the iron found in pyrite, but they also contain silicone and oxygen, which must be removed and. Web pyrite, or iron disulfide, is the most common sulfide mineral on the earth’s surface and is widespread through the geological record. Sulfur, which.
It forms in a variety of geological settings through several processes. Over time, iron sulfide minerals crystallize, resulting in pyrite’s formation. This then reacts with an iron compound forming pyrite. The specimen’s size can vary. Redox sensitive trace elements in pyrite, including nodules, are increasingly used to infer the chemical conditions of ancient oceans—but considerable uncertainty remains regarding the mechanism.
Pyrite is called fool’s gold; Web the relatively rapid formation of pyrite framboids explains how pyrite infills and preserves soft tissues before cell lysis and before deformation through burial has been initiated. Healing properties & everyday uses. It has a chemical composition of iron sulfide (fes 2) and is the most common sulfide mineral. Bacteria breaks down the organics forming.
Web pyrite typically forms in cubic crystals, often accompanied by striations on the crystal faces. One of those forms is pyritic sulfur, which occurs as iron sulfide or. Web the formation of pyrite crystals depends mainly on the iron content of the sediment. Redox sensitive trace elements in pyrite, including nodules, are increasingly used to infer the chemical conditions of.
Often mistaken for gold due to its metallic luster, pyrite has a playful nickname but holds real value. The specimen’s size can vary. It has a chemical composition of iron sulfide (fes 2) and is the most common sulfide mineral. Bacterial sulphate reduction produces bisulphide. Healing properties & everyday uses.
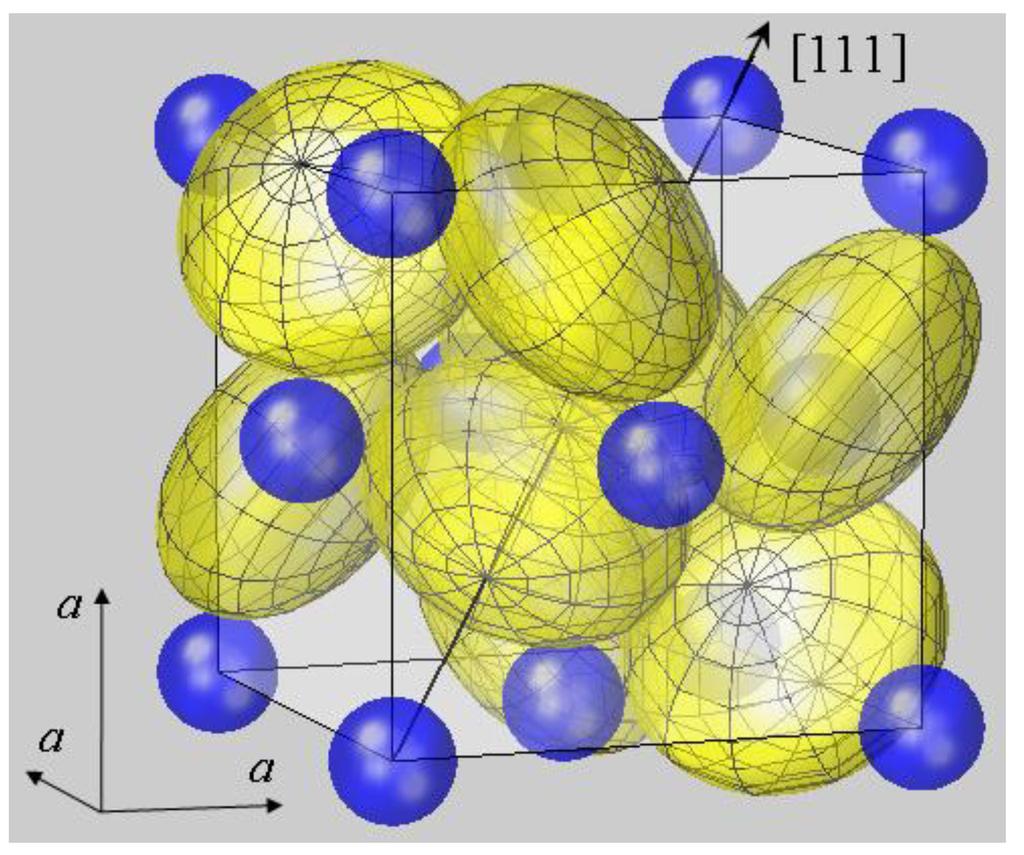
How Does Pyrite Form - Doug meek / getty images. If there is iron present, iron sulphide crystals begin to grow. To the novice its colour is deceptively similar to that of a gold nugget. Web pyrite, or iron disulfide, is the most common sulfide mineral on the earth’s surface and is widespread through the geological record. Web the formation of pyrite crystals depends mainly on the iron content of the sediment. These crystals can also be octahedral and pyritohedral in shape. Redox sensitive trace elements in pyrite, including nodules, are increasingly used to infer the chemical conditions of ancient oceans—but considerable uncertainty remains regarding the mechanism and timing of nodule formation. Often mistaken for gold due to its metallic luster, pyrite has a playful nickname but holds real value. Web pyrite ( fesx2 f e s x 2) forms cubic crystals, like these ones: It has a chemical composition of iron sulfide (fes 2) and is the most common sulfide mineral.
Web the formation of pyrite starts with chlorite iron deposits within the ground. It has a chemical composition of iron sulfide (fes 2) and is the most common sulfide mineral. Redox sensitive trace elements in pyrite, including nodules, are increasingly used to infer the chemical conditions of ancient oceans—but considerable uncertainty remains regarding the mechanism and timing of nodule formation. The name comes from the greek word pyr, “fire,” because pyrite emits sparks when struck by metal. This then reacts with an iron compound forming pyrite.
This remarkable mineral often occurs in sedimentary rocks, coal beds, shale, and sometimes in hydrothermal veins or igneous rocks. Doug meek / getty images. To the novice its colour is deceptively similar to that of a gold nugget. Web pyrite, a naturally occurring iron disulfide mineral.
It is mined as a source of sulfur and iron as well as of the impurities gold and copper. Iron sulfide, sometimes containing small amounts of cobalt, nickel, silver, and gold. Bacteria breaks down the organics forming bisulphide, the s2 component of pyrite.
Web pyrite ( fesx2 f e s x 2) forms cubic crystals, like these ones: Web the formation of pyrite crystals depends mainly on the iron content of the sediment. Pyrite forms under reducing conditions, typically in environments with low oxygen levels, high sulfur content, and abundant iron.
Web The Formation Of Pyrite Starts With Chlorite Iron Deposits Within The Ground.
Often mistaken for gold due to its metallic luster, pyrite has a playful nickname but holds real value. Web where it is found in igneous rocks, it may have been formed from magma in which the minerals were heated into a melted mass, and then the various minerals separated out at different temperatures, forming crystals as it cooled; Bacteria breaks down the organics forming bisulphide, the s2 component of pyrite. Howstuffworks | aug 25, 2023.
The Specimen’s Size Can Vary.
If there is iron present, iron sulphide crystals begin to grow. This remarkable mineral often occurs in sedimentary rocks, coal beds, shale, and sometimes in hydrothermal veins or igneous rocks. To the novice its colour is deceptively similar to that of a gold nugget. This then reacts with an iron compound forming pyrite.
Sulfur, Which Can Occur In Coal And Is A Pollutant When Burned, Occurs In Three Forms.
One of those forms is pyritic sulfur, which occurs as iron sulfide or. Web pyrite, also known as “fool’s gold,” is a common iron sulfide mineral with the chemical formula fes2. It has a chemical composition of iron sulfide (fes 2) and is the most common sulfide mineral. Web pyrite forms large bodies in moderate to high temperature hydrothermal deposits and in contact metamorphic ore deposits, is an accessory in many igneous rocks, and is common in sedimentary beds and metamorphosed sediments.
Pyrite Forms Under Reducing Conditions, Typically In Environments With Low Oxygen Levels, High Sulfur Content, And Abundant Iron.
It forms at high and low temperatures and occurs, usually in small quantities, in igneous, metamorphic, and sedimentary rocks worldwide. Healing properties & everyday uses. Web pyrite ( fesx2 f e s x 2) forms cubic crystals, like these ones: Web it forms when iron and sulfur combine in specific geological conditions that typically involve the presence of water and oxygen.